随着全球人口的增长和城市化的发展,对能源的需求也日益增加。如何高效的利用清洁无污染的能源,如风能,太阳能和生物能等,也一直受到人们的关注。而储存这些清洁能源设备,则是利用这些能源的关键。在目前的能量储存技术中,二次电化学储能设备由于其可被大规模装备和智能化管理,因此被认为是最有前景的能量存储设备。在这些二次能量存储设备中,锂离子电池早已在各个领域中获得了广泛的应用;除锂离子电池之外,钠离子,镁离子和铝离子电池以及超级电容器也有了相关报道。那么选择和设计合适的电极材料,则是影响二次能量存储设备的性能的核心问题。
一些一维纳米材料,比如纳米线,纳米纤维,纳米带和纳米管等,能够有效的缓解电极材料在循环过程中的应力问题,遏制电极材料的体积膨胀效应。而多孔结构可以使电极材料和电解液充分接触,有利于电荷传递和离子扩散。结合上述两种电极材料的优点,设计和制备多孔结构的一维材料,为解决电极材料面临的问题提供了一种新的思路。近日,武汉理工的麦立强教授和加州大学洛杉矶分校的Bruce Dunn教授带领的研究团队总结并归纳了一维多孔材料的设计思路,合成方法及其在电化学领域的应用,相关研究发表在《先进材料》(Advanced Materials)。
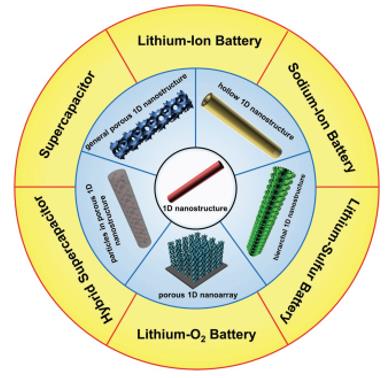
设计材料的思路分为两种,一种是“top-down”(自上而下),另一种是“bottom-up” (自下而上)。而对于多孔一维纳米材料,则是结合着两种方法,首先是利用“top-down”来制备一维纳米材料的骨架结构,然后利用“bottom-up”来合成孔洞结构。常用的合成一维多孔材料的方法有(1)利用静电纺丝技术(图3),(2)利用水热或是溶剂热等液相合成方法(图6),(3)利用模板合成方法(图9),(4)利用气相沉积或者电沉积的化学沉积合成方法(图12),(5)化学刻蚀的方法(图14)。
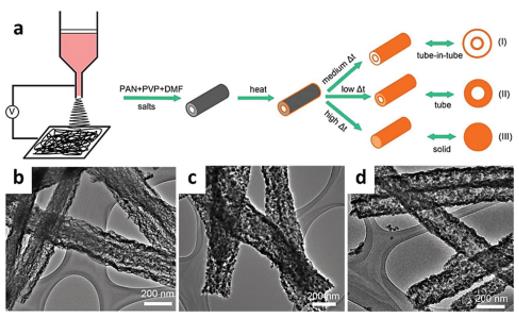
Figure 3. a) Illustration showing nanostructure control using heating rate. SEM images of tube-in-tube nanostructures (b), porous nanotubes (c) and porous nanowires (d). Reproduced with permission.[74] Copyright 2015, American Chemical Society.
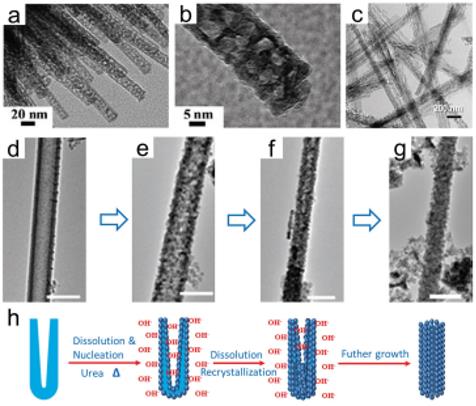
Figure 6. a,b) TEM images of urchin-like mesoporous Co(OH)2 nanowire arrays, reproduced with permission.[104] Copyright 2010, Royal Society of Chemistry. c) TEM image of LT-TiO2 nanowires, reproduced with permission.[110] Copyright 2010, IOP Publishing. d–g) Morphology transformation from TiO2 nanotube to porous TiO2 nanowire at different reaction times: (d) 0 h, (e) 2 h, (f) 4 h, (g) 6 h and (h) corresponding schematic illustration, reproduced with permission.[111] Copyright 2013, Elsevier.

Figure 9. a–d) Schematic of the process for fabricating porous Pt-Co alloy nanowires. (e,f) TEM images of porous Pt-Co alloy nanowires, scale bar is 50 nm. Figures a–f reproduced with permission.[131] Copyright 2009, American Chemical Society. g) Fabrication process of porous nanowires via a dual template technique, reproduced with permission.[145] Copyright 2003, American Chemical Society.

Figure 12. a) Illustration of the template-assisted electrochemical preparation of asymmetric porous gold nanowires. b) TEM image of porous stepcone nanostructures prepared by varying Au/Ag ratios in plating solutions. c) TEM image of porous nano-barbell nanostructures. Figures a–c reproduced with permission.[173] Copyright 2007, American Chemical Society. d) SEM image of a porous and spiral PdCu nanowire bundle, reproduced with permission.[174] Copyright 2013, American Chemical Society. e) Schematic of the hydrothermal-electrodeposition of hierarchical porous core/shell nanowire arrays. f) SEM image of core/shell Co3O4@Co(OH)2 nanowire. Figures e,f reproduced with permission.[117] Copyright 2012, American Chemical Society.
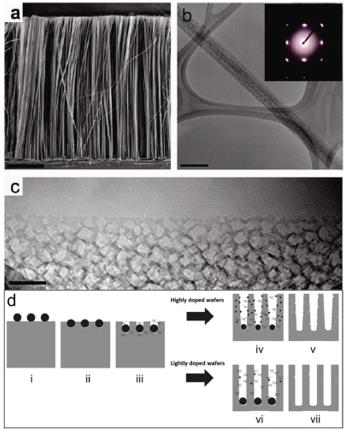
Figure 14. a) SEM and b,c) TEM images of porous silicon nanowires synthesized from highly doped p-type Si wafers (inset in b is corresponding SAED pattern), the scale bar is 10 mm, 200 nm, and 50 nm in a–c, respectively, reproduced with permission.[189] Copyright 2009, American Chemical Society. d) Schematic of the formation of porous and nanoporous Si nanowire arrays from highly or lightly doped wafers through a two-step silver assisted etching method, reproduced with permission.[198] Copyright 2011, American Chemical Society.
一维多孔材料由于其独特的优点,在锂离子电池,钠离子电池,锂-硫电池,锂-氧气电池等领域也得到了广泛的研究与应用。在锂离子电池,钠离子电池中(图17,18),一维多孔材料能够有效解体积膨胀效应;在锂-硫电池,锂-氧气电池中(图20,21),一维多孔材料能够提供更多的反应传输通道;而在超级电容器中(图23),一维多孔材料能够提供更大的比表面积,改善其电化学性能。
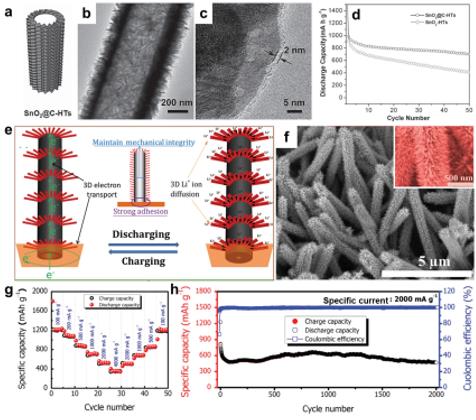
Figure 17. a) Schematic, (b) TEM and (c) HRTEM images of SnO2@C-HTs. d) Cycling performance of SnO2@C-HTs and SnO2-HTs at 200 mA g−1 for LIBs. Figures a–d reproduced with permission.[59] Copyright 2013, WILEY-VCH. e) Schematic of hierarchical tubular transition metal oxide core/shell heterostructures with 3D Li+/electron transport pathways. f) SEM image, (g) rate performance, and (h) cycling performance of hierarchical tubular CuO/CoO core/shell heterostructures. Figures e–h reproduced with permission.[215] Copyright 2015, Elsevier.
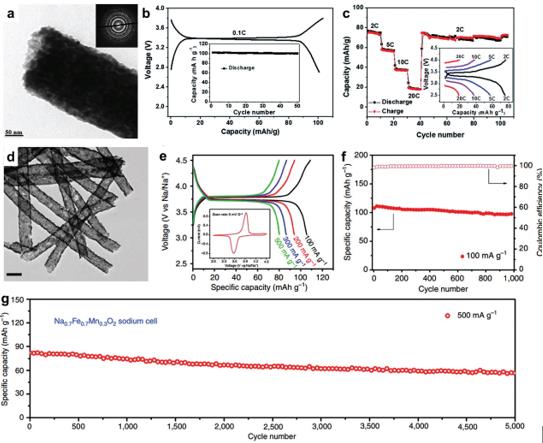
Figure 18. a) TEM image, (b) frst charge-discharge curves and cycling performance at 0.1C and (c) rate performance of Na3V2(PO4)3/C porous nanofbers, reproduced with permission.[217] Copyright 2014, Royal Society of Chemistry. d) TEM image (scale bar is 200 nm), (e) charge–discharge curves (inset showing CV curve at 5 mV s–1) and (f,g) cycling performance of Na0.7Fe0.7Mn0.3O2 mesoporous nanotubes, reproduced with permission.[66] Copyright 2015, Nature Publishing Group.
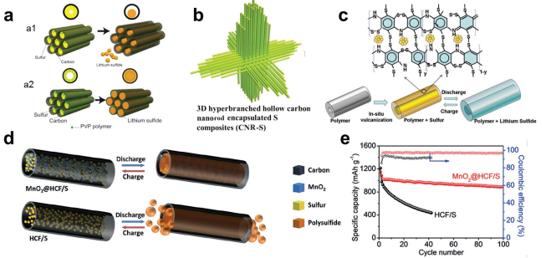
Figure 20. a) Schematic of structural changes in hollow carbon nanofber with encapsulated sulfur (a1) without PVP and (a2) with PVP, reproduced with permission.[250] Copyright 2013, American Chemical Society. b) Schematic of 3D hyperbranched hollow carbon nanorod-sulfur (CNR-S) nanocomposites, reproduced with permission.[251] Copyright 2014, Wiley-VCH. c) Drawing of SPANI-NT/S composite structure, reproduced with permission.[253] Copyright 2012, Wiley-VCH. d) Schematic and (e) electrochemical performance of MnO2@HCFs/S and HCF/S, reproduced with permission.[62] Copyright 2015, Wiley-VCH.

Figure 21. a) SEM and (b) TEM images of porous Co3O4 nanowires. c) The frst discharge curves of Li–O2 batteries at 0.1 A g–1. Figures a–c reproduced with permission.[271] Copyright 2015, Springer. d) Schematic of La0.5Sr0.5CoO2.91 porous nanowires and SEM image (inset). e) The frst discharge curve of Li–O2 batteries with La0.5Sr0.5CoO2.91 porous nanowires at 50 mA g–1. Figures d,e reproduced with permission.[68] Copyright 2012, the National Academy of Sciences. f) Schematic of oxygen diffusion in porous La0.75Sr0.25MnO3 nanotubes. g) Terminal discharge voltage vs. cycle number of Li-O2 batteries with and without porous La0.75Sr0.25MnO3 nanotubes at 0.15 mA cm–2. Figures f,g reproduced with permission.[276] Copyright 2013, Wiley-VCH.

Figure 23. a) HRTEM image (b) CV curves, and (c) specifc capacitances of N-doped CNFs, reproduced with permission.[289] Copyright 2012, American Chemical Society. (d) Illustration, (e) cycling performance and (f) Ragone plots of mesoporous CoO@Ppy nanowire arrays, reproduced with permission.[307] Copyright 2013, American Chemical Society. g) Illustration, (h) CV curves and (i) rate performance of MnO2/Mn/MnO2 SNTAs and MnO2 NTAs, reproduced with permission.[65] Copyright 2012, American Chemical Society.
伴随着具有结构可控特点的一维多孔纳米材料的发展,也同时促进了能源储存材料的发展。目前一维多孔材料的合成制备方法复杂,成本高昂,为了实现一维多孔材料的广泛应用,还有很多理论上和实践上的工作需要去探究。但是,这种一维多孔材料为解决电极材料的面临的问题提供了一种新的方法,也为日后设计高性能的能源储存设备提供了一种新的思路。
本研究由武汉理工的麦立强教授和加州大学洛杉矶分校的Bruce Dunn教授带领完成,于2017年发表在《先进材料》(Advanced Materials)上。
论文信息:Wei Q, Xiong F, Tan S, et al. Energy Storage: Porous One‐Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage (Adv. Mater. 20/2017) [J]. Advanced Materials, 2017, 29(20).
